Roche (RHHBY) Gets Positive CHMP Recommendation for Its Drugs
Roche RHHBY announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended a label expansion of ophthalmology drug Vabysmo (faricimab).
The CHMP gave a positive opinion for the label extension of Vabysmo for a third indication — treatment of visual impairment due to macular edema secondary to retinal vein occlusion (RVO).
The European Commission is expected to give a final decision shortly.
The positive CHMP opinion is based on full 72-week data from the late-stage BALATON and COMINO studies evaluating Vabysmo in more than 1,200 people with macular edema due to branch and central RVO.
Data from these studies showed Vabysmo demonstrated early and sustained vision improvements non-inferior to Regeneron’s REGN Eylea (aflibercept), along with robust retinal drying. Vabysmo was well tolerated and the safety profile was consistent with the previous studies.
Per Roche, current available treatments for RVO are typically given every one to two months.
Please note that Vabsymo is already approved in several countries around the world for people living with neovascular or ‘wet’ age-related macular degeneration (nAMD) and diabetic macular edema (DME). In October 2023, the FDA approved Vabysmo for RVO.
A potential approval will make Vabysmo the first and only bispecific antibody treatment available for nearly 1 million people with RVO in the European Union.
Roche has a broad ongoing development program for Vabysmo. The program includes AVONELLE-X, an extension study of TENAYA and LUCERNE, evaluating the long-term safety and tolerability of Vabysmo in nAMD and RHONE-X, an extension study of YOSEMITE and RHINE evaluating the long-term safety and tolerability of Vabysmo in DME.
Concurrently, Roche announced that the CHMP has adopted a positive opinion for PiaSky (crovalimab) for the treatment of paroxysmal nocturnal haemoglobinuria (PNH).
The CHMP has recommended PiaSky, a novel recycling monoclonal antibody that inhibits the complement protein C5, for use in adults and adolescents (12 years of age or older with a weight of 40 kg) who are either new to or have been previously treated with C5 inhibitors.
A potential approval will make PiaSky the first monthly subcutaneous (SC) treatment for PNH in the European Union, with the option to self-administer following adequate training.
The recommendation is based on the COMMODORE 2 study results, where PiaSky SC given every month demonstrated equivalent disease control and comparable safety to intravenous Soliris given every two weeks.
Roche’s shares have lost 4.3% year to date against the industry’s growth of 21.2%.
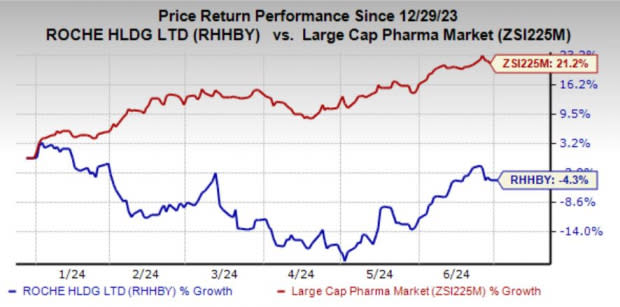
Image Source: Zacks Investment Research
Approval of new drugs and label expansion of the existing ones should bode well for Roche in this scenario.
Drugs like Vabysmo, Ocrevus, Hemlibra and Polivy fuel Roche’s top line as the company looks to fill up the dent in revenues caused by a decline in COVID-19-related sales. Competition from biosimilars for established drugs like Avastin, MabThera/Rituxan and Herceptin continues to hurt sales.
The uptake of Vabysmo has been outstanding. Eylea's sales have been under pressure due to competition from Vabysmo. To counter the decline in Eylea sales, Regeneron developed a higher dose of the drug. The initial uptake of Eylea HD is encouraging as Eylea patients transition to the higher dose.
Zacks Rank & Other Stocks to Consider
Roche currently carries a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the healthcare sector are ALX Oncology Holdings ALXO and Minerva Neurosciences, Inc. NERV, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, the Zacks Consensus Estimate for ALX Oncology’s 2024 loss per share has narrowed from $3.33 to $2.89. During the same period, the consensus estimate for 2025 loss per share has narrowed from $2.85 to $2.73.
ALX Oncology beat on earnings in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 8.83%.
In the past 60 days, estimates for Minerva Neurosciences’ 2024 loss per share have narrowed from $3.57 to $1.89. The loss per share estimate for 2025 has narrowed from $4.54 to $3.60 during the same time frame.
NERV’s earnings beat estimates in one of the trailing four quarters and missed the same in the other three, the average negative surprise being 54.43%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Minerva Neurosciences, Inc (NERV) : Free Stock Analysis Report
ALX Oncology Holdings Inc. (ALXO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 