Ultragenyx's (RARE) IND for mRNA Therapy, UX053 Cleared by FDA
Ultragenyx Pharmaceutical Inc. RARE announced that the FDA has cleared the investigational new drug or IND application, for its lead mRNA therapy, UX053, which is being developed for the treatment of Glycogen Storage Disease Type III (“GSDIII”). Currently there is no approved treatment option available for this indication.
Notably, GSDIII is caused by a glycogen-debranching enzyme deficiency resulting in glycogen accumulation in the liver and muscle.
The company plans to begin enrollment in a phase I/II study evaluating UX053 for the given indication in the second half of 2021.
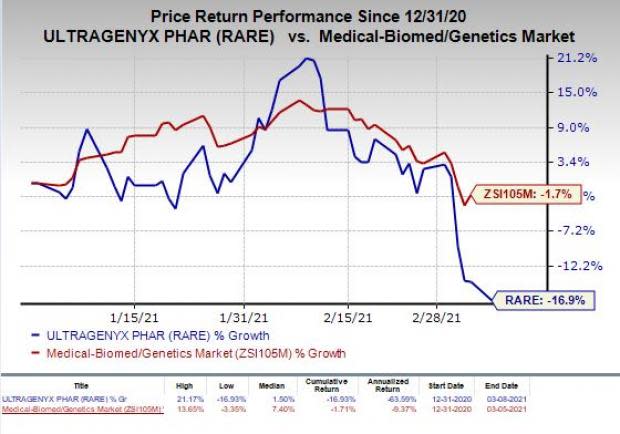
Shares of Ultragenyx have declined 16.9% so far this year compared with the industry’s decrease of 1.7%.
Per the press release, the two-part study will investigate the safety, tolerability and efficacy of UX053 in adults with GSDIII. The part 1 of the phase I/II study will enroll up to 12 patients who will receive a single ascending dose of UX053. The part 2 of the above study is a placebo-controlled multi-ascending study of 5 doses of UX053 looking to enroll around 16 patients across four cohorts randomized 3:1 to UX053 or placebo.
Notably, Ultragenyx is developing various mRNA therapies in the preclinical studies for addressing undisclosed indications. Out of these, UX053 is the most advanced and the first mRNA program to enter clinical development for rare genetic diseases.
Please note that Ultragenyx has a collaboration agreement with California-based biotech Arcturus Therapeutics ARCT for developing the above-mentioned mRNA therapies.
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #4 (Sell).
Better-ranked stocks in the biotech sector include Stoke Therapeutics, Inc. STOK and Repligen Corporation RGEN, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Stoke Therapeutics’ loss per share estimates have narrowed 2.1% for 2021 over the past 60 days.
Repligen’s earnings estimates have been revised 15.1% upward for 2021 over the past 60 days.
These Stocks Are Poised to Soar Past the Pandemic
The COVID-19 outbreak has shifted consumer behavior dramatically, and a handful of high-tech companies have stepped up to keep America running. Right now, investors in these companies have a shot at serious profits. For example, Zoom jumped 108.5% in less than 4 months while most other stocks were sinking.
Our research shows that 5 cutting-edge stocks could skyrocket from the exponential increase in demand for “stay at home” technologies. This could be one of the biggest buying opportunities of this decade, especially for those who get in early.
See the 5 high-tech stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Repligen Corporation (RGEN) : Free Stock Analysis Report
Ultragenyx Pharmaceutical Inc. (RARE) : Free Stock Analysis Report
Stoke Therapeutics, Inc. (STOK) : Free Stock Analysis Report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 