Vertex's (VRTX) Non-Opioid Pain Drug Meets Primary Goal
Vertex Pharmaceuticals VRTX announced positive results from three late-stage studies on its investigational orally-administered non-opioid pain medicine, VX-548, to treat moderate-to-severe acute pain.
The company evaluated VX-548, a novel first-in-class NaV1.8 inhibitor, in two pivotal phase III acute pain studies — one following bunionectomy surgery and the other following abdominoplasty surgery. The studies achieved their primary endpoint — treatment with VX-548 showed a significant reduction in pain intensity across a 48-hour period compared with placebo.
Both studies failed to meet a key secondary goal of the superiority of VX-548 over the combination of opioid drug hydrocodone bitrate and acetaminophen (HB/APAP) in reducing pain.
Vertex also evaluated VX-548 for up to 14 days in a single-arm late-stage study across a broad range of other surgical and non-surgical acute pain conditions. Data from the study showed that around 83% of study participants rated the drug as ‘good, very good, or excellent’ in treating pain.
Based on the above data, management intends to submit a new drug application (NDA) with the FDA for VX-548 across a broad label in moderate-to-severe acute pain by mid-2024.
Shares of Vertex rose 2.4% on Tuesday following the news announcement. Currently, opioids are the standard of care for treating acute pain. Though they can be very effective, they carry severe potential risks of addiction and abuse. While the studies failed to achieve superiority over opioid medications in treating pain, an area with limited treatment options, they did demonstrate the drug’s effectiveness as an alternative to opioids.
In the past year, the stock has risen 38.1% against the industry’s 12.3% decline.

Image Source: Zacks Investment Research
Last month, Vertex reported positive data from a mid-stage study on VX-548 in painful diabetic peripheralneuropathy (DPN), a type of neuropathic pain caused by diabetes. Treatment with the drug showed a statistically significant and clinically meaningful reduction in pain intensity.
Based on the above result, Vertex intends to move VX-548 to pivotal development for DPN. The company also initiated a phase II study on VX-548 in patients with painful lumbosacral radiculopathy, another form of peripheral neuropathic pain (caused by damage to nerves).
Though Vertex enjoys a dominant position in the cystic fibrosis (CF) market, it has seen success in the development of its non-CF pipeline candidates lately.
Last month, the FDA approved Vertex and partner CRISPR Therapeutics’ CRSP Casgevy (exagamglogene autotemcel) for the treatment of sickle cell disease (SCD) for patients aged 12 years and older with recurrent vaso-occlusive crises. Following this approval, Casgevy became the first gene therapy utilizing the Nobel prize-winning CRISPR technology. This technology can selectively delete, modify or correct a disease-causing abnormality in a specific DNA segment.
Earlier this month, Vertex/CRISPR announced that the FDA expanded the label of their one-shot gene therapy Casgevy to treat transfusion-dependent beta thalassemia (TDT) in patients aged 12 years and older.
Vertex Pharmaceuticals Incorporated Price
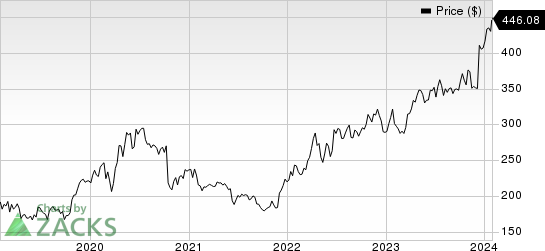
Vertex Pharmaceuticals Incorporated price | Vertex Pharmaceuticals Incorporated Quote
Zacks Rank & Key Picks
While Vertex carries a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the overall healthcare sector include Anavex Life Sciences AVXL and Sarepta Therapeutics SRPT. While Anavex sports a Zacks Rank #1 (Strong Buy), Sarepta carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, estimates for Anavex Life Sciences’ 2023 loss per share have improved from 57 cents to 53 cents. Shares of Anavex lost 42.1% in the year-to-date period.
Anavex beat earnings estimates in each of the last four quarters, delivering an earnings surprise of 15.80% on average. In the last reported quarter, Anavex’s earnings beat estimates by 25.00%.
In the past 30 days, Sarepta’s loss estimates for 2023 have improved from a loss of $6.80 per share to $6.57 per share. During the same period, earnings estimates per share for 2024 have risen from $1.71 to $2.14. Sarepta’s shares have lost 4.7% in the past year.
Sarepta’s earnings beat estimates in each of the last four quarters, delivering an average surprise of 48.67%. In the last reported quarter, Sarepta’s earnings beat estimates by 72.29%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Anavex Life Sciences Corp. (AVXL) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 