Viking (VKTX) Announces Clinical Data for Metabolic Disorders
Shares of Viking Therapeutics VKTX have surged almost 70% on Mar 28, after it announced positive clinical data of VK2735, a novel dual agonist of the glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors for treating various metabolic disorders. The company has also initiated an early-stage extension study on VK2735.
The study demonstrated up to 6% placebo-adjusted mean weight reduction (7.8% from baseline) from the drug, observed after 28 days of usage.
The phase I clinical study on VK2735 evaluated a single ascending dose (SAD) and multiple ascending doses (MAD). In the SAD portion of the study, the drug demonstrated promising safety and tolerability, as well as a predictable pharmacokinetic profile. In the study, a single injection of VK2735 demonstrated a half-life of approximately 170 to 250 hours, a Tmax (time to reach maximum plasma concentration) between 75 and 90 hours, and excellent therapeutic exposures.
In the 28-day MAD portion of the study, VK2735 demonstrated encouraging tolerability and positive signs of clinical activity. All cohorts receiving the drug showed reductions in mean body weight from baseline, ranging up to 7.8%. They also demonstrated reductions in mean body weight relative to placebo, ranging up to 6.0%. Statistically significant differences compared to placebo were maintained or improved at the day 43 follow-up time point, 21 days after the last dose of VK2735 was administered.
Shares of Viking have surged 358.6% in the past year against the industry's 16.4% decline.
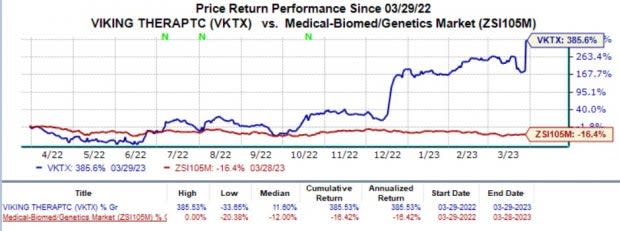
Image Source: Zacks Investment Research
Management believes tolerability data from the said study indicate that higher doses may be achieved with longer titration windows. Based on the phase I results, VKTX plans to initiate a phase II study by mid-2023 of VK2735 to treat patients with obesity. The objective of the study will be to evaluate the dose escalation of VK2735 in obese patients.
Viking is also extending the phase I clinical study to evaluate the oral formulation of GLP-1 and GIP receptors. The study's primary objective will be to evaluate the safety and tolerability of VK2735 administered as an oral tablet once daily for 28 days. The secondary objective will be to evaluate the pharmacokinetics of orally administered VK2735 in healthy patients. The data from the extended study is expected in the second half of 2023.
The company’s other clinical study includes VK2809, a novel, orally available, small molecule selective thyroid hormone receptor beta agonist for the potential treatment of lipid and metabolic disorders. The drug is currently being evaluated in a phase IIb study for the treatment of biopsy-confirmed non-alcoholic steatohepatitis and fibrosis.
Viking Therapeutics, Inc. Price and Consensus
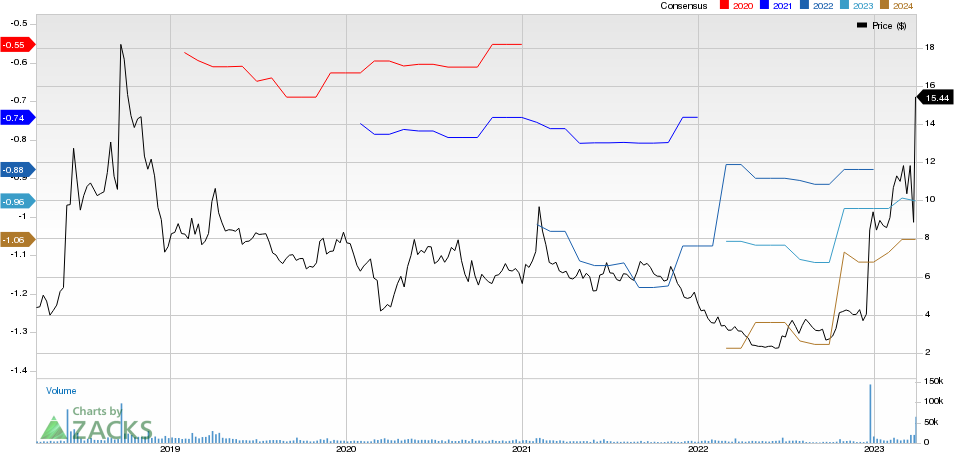
Viking Therapeutics, Inc. price-consensus-chart | Viking Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Currently, Viking has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same sector are Novo Nordisk NVO, Kala Pharmaceuticals KALA and Jasper Therapeutics JSPR. Novo Nordisk sports a Zacks Rank #1 (Strong Buy), and Kala Pharmaceuticals and Jasper Therapeutics carry a Zacks Rank #2 (Buy) at present. You can see the complete list of today's Zacks #1 Rank stocks here.
Earnings per share estimates for NVO have increased from $4.18 to $4.49 for 2023 and from $4.38 to $5.29 for 2024 in the past 60 days. The company’s shares have gained 41% in the past year.
NVO's earnings beat estimates in three of the last four quarters and missed the mark in one, the average surprise being 3%.
Loss per share estimates for Kala Pharmaceuticals have narrowed from $19.67 to $15.35 for 2023 and from $14.41 to $13.12 for 2024 in the past 60 days. KALA's shares have plunged 79% in the past year.
The company’s earnings beat estimates in two of the last four quarters and missed the mark in the other two, the average surprise being 11.56%.
Loss per share estimates for Jasper Therapeutics have narrowed from $1.42 to 61 cents for 2023 and from $1.6 to 59 cents for 2024 in the past 60 days. JSPR's shares have plunged 45.8% in the past year.
The company’s earnings beat estimates in three of the last four quarters and met the mark in one, the average surprise being 3.51%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Kala Pharmaceuticals, Inc. (KALA) : Free Stock Analysis Report
Jasper Therapeutics, Inc. (JSPR) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 