Adaptimmune Therapeutics plc (ADAP)
NasdaqGS - NasdaqGS Real-time price. Currency in USD
Add to watchlist
At close: 04:00PM EDT
After hours:
| Previous close | 1.1900 |
| Open | 1.2200 |
| Bid | 1.1800 x 600 |
| Ask | 1.2100 x 600 |
| Day's range | 1.1722 - 1.2400 |
| 52-week range | 0.4200 - 2.0500 |
| Volume | |
| Avg. volume | 1,593,456 |
| Market cap | 304.119M |
| Beta (5Y monthly) | 2.38 |
| PE ratio (TTM) | N/A |
| EPS (TTM) | -0.5400 |
| Earnings date | 15 May 2024 |
| Forward dividend & yield | N/A (N/A) |
| Ex-dividend date | N/A |
| 1y target est | 3.50 |
 Zacks
ZacksAdaptimmune (ADAP) Down on End of Collaboration With Roche
Adaptimmune's (ADAP) shares plummet as the company;s collaboration to develop and commercialize allogeneic cell therapies with Roche gets terminated.
 Simply Wall St.
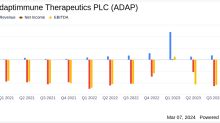
Simply Wall St.Adaptimmune Therapeutics Full Year 2023 Earnings: EPS Beats Expectations, Revenues Lag
Adaptimmune Therapeutics ( NASDAQ:ADAP ) Full Year 2023 Results Key Financial Results Revenue: US$60.3m (up 122% from...
 GuruFocus.com
GuruFocus.comAdaptimmune Therapeutics PLC Reports Q4 and Full Year 2023 Financial Results
Key Financial Highlights and Business Updates