AstraZeneca/Oxford vaccine shows 'strong immune response' in all ages
Watch: Oxford vaccine produces strong immune response in older adults, early results show
Early results from the latest stage of testing for AstraZeneca (AZN.L) and the University of Oxford’s possible COVID-19 vaccine show a “strong immune response... across all age groups.”
AstraZeneca on Thursday said interim results from its Phase II/III trial showed patients given the vaccine exhibited a “robust immune response.” Crucially, participants of all ages showed an immune system reaction.
“It is essential that a COVID-19 vaccine can be effective across a broad age range, particularly in older individuals where they are disproportionately at risk of severe COVID-19 disease,” said Mene Pangalos, executive vice president of BioPharmaceuticals R&D at AstraZeneca.
Almost 13,000 people in the UK are taking part in the trial of the vaccine and 60,000 people around the world are trialling it. The vaccine candidate is being developed in partnership with the University of Oxford.
Patients who received the vaccine experienced some “mild, local and systemic reactions,” such as pain in the injection site and headaches. Older patients who received the vaccine showed less symptoms than young participants.
Professor Andrew Pollard, the head of Oxford’s vaccine trial team, told BBC Radio 4’s Today programme: “Adults tend to feel a bit ropey the day after they have been vaccinated.”
AstraZeneca said it was inconclusive whether the vaccine generated a lasting immunity to COVID-19 for patients who receive it.
READ MORE: Oxford's coronavirus vaccine is safe and produces immune response among elderly, study suggests
UK health secretary Matt Hancock said the results were a “really encouraging set of findings.” Earlier this year Britain signed a deal to buy 100 million doses of the AstraZeneca and University of Oxford vaccine if successful.
Dr Michael Tildesley, who sits on a Sage sub-group, told BBC Breakfast the vaccine could “hopefully [be] one of the key game changers.”
Professor Pollard told the Today programme he was “absolutely delighted” with the interim results.
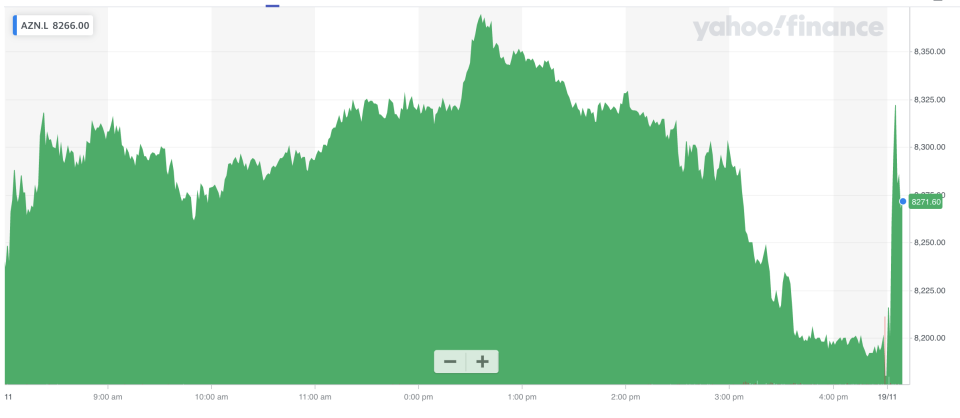
Shares in AstraZeneca rose over 1% at the open in London.
The results add to the recent mood of optimism around hopes for a COVID-19 vaccine that could end the pandemic.
Last week Pfizer (PFE) and BioNTech (BNTX) announced their vaccine candidate was 90% effective in trials. They followed with a further update yesterday saying final analysis suggested it was in fact 95% effective.
This week US biotech company Moderna (MRNA) said its vaccine was 94.5% effective in trials.
“Overall the newsflow has been incredibly positive in the last couple of weeks,” said Jim Reid, a senior investment strategist at Deutsche Bank.

 Yahoo Finance
Yahoo Finance 