Vertex (VRTX) NDA for Vanza Triple Therapy Accepted by FDA
Vertex Pharmaceuticals Incorporated VRTX announced that the FDA has accepted its new drug application (“NDA”) for vanza triple therapy for treating people with cystic fibrosis (“CF”) aged six years and above.
The company is seeking FDA’s nod for vanza triple in CF aged six years and older who have at least one F508del mutation or another responsive mutation in the CF transmembrane conductance regulator (CFTR) gene responsive to the vanza triple.
Vanza triple is a combination of vanzacaftor, a CFTR potentiator, deutivacaftor, a CFTR corrector and tezacaftor.
Vanza triple is a new once-a-day oral combination medicine that has the potential for enhanced patient benefit than Trikafta patients and can potentially treat CF patients who have discontinued Trikafta or other Vertex CF medicines. It can also improve dosing (once daily) and lower the royalty burden.
With the FDA granting a priority review to the NDA, a decision from the regulatory body is expected on Jan 2, 2025.
Simultaneously, the European Medicines Agency has also validated the marketing authorization application for vanza triple for CF patients aged six years and older.
VRTX filed regulatory applications for vanza triple for treating people with CF aged six years and older using a priority voucher in the United States and in the European Union in May.
Shares of Vertex have rallied 16.5% so far this year against the industry’s decline of 6.3%.
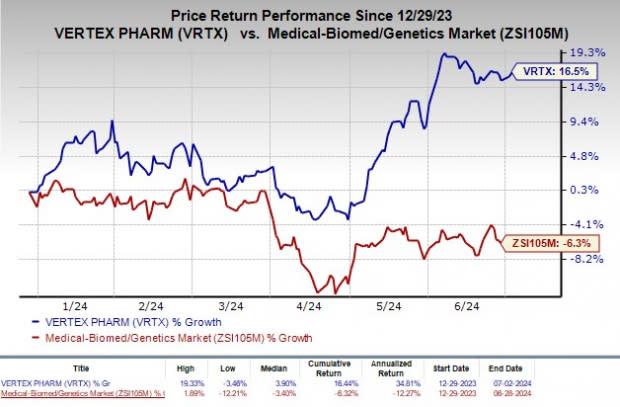
Image Source: Zacks Investment Research
VRTX has also submitted regulatory filings for vanza triple in Canada, Australia, Switzerland and the United Kingdom.
Vertex holds a dominant position in the CF market. The company’s CF sales continue to grow, driven by its triple therapy, Trikafta (marketed as Kaftrio in Europe).
Trikafta/Kaftrio generated revenues of $2.48 billion in the first quarter of 2024, up 18.5% on a year-over-year basis. Sales are being driven by the continued robust performance of the drug in and outside the United States, fueled by label expansions to younger age groups.
A potential approval for vanza triple is likely to boost Vertex’s CF franchisee sales further in 2024 and beyond.
Zacks Rank & Stocks to Consider
Vertex currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Acrivon Therapeutics, Inc. ACRV, Aligos Therapeutics, Inc. ALGS and RAPT Therapeutics, Inc. RAPT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Acrivon Therapeutics’ 2024 loss per share have narrowed from $3.05 to $2.47. Loss per share estimates for 2025 have narrowed from $3.04 to $2.55. Year to date, ACRV shares have rallied 23.6%.
ACRV’s earnings beat estimates in three of the trailing four quarters and missed the same on the remaining one occasion, the average surprise being 3.56%.
In the past 60 days, estimates for Aligos Therapeutics’ 2024 loss per share have narrowed from 84 cents to 73 cents, while loss per share estimates for 2025 have narrowed from 82 cents to 71 cents. Year to date, ALGS shares have declined 39.8%.
Aligos’ earnings beat estimates in three of the trailing four quarters and missed the same on the remaining occasion, the average surprise being 7.83%.
In the past 60 days, estimates for RAPT Therapeutics’ 2024 loss per share have narrowed from $3.19 to $2.93. Loss per share estimates for 2025 have narrowed from $2.40 to $2.05. Year to date, RAPT shares have declined 88.8%.
RAPT’s earnings beat estimates in two of the trailing four quarters while missing the same on the remaining two occasions, the average surprise being 3.19%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
Rapt Therapeutics (RAPT) : Free Stock Analysis Report
Aligos Therapeutics, Inc. (ALGS) : Free Stock Analysis Report
Acrivon Therapeutics, Inc. (ACRV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 